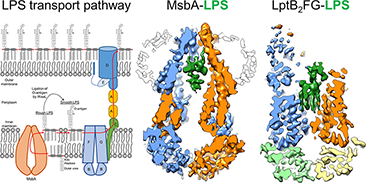
Antibiotic resistance has become a global health threat. Gram-negative bacteria are among the most difficult pathogen to combat, mainly due to their unique “outer membrane” which prevents most antibiotics from entering the cells. Lipopolysaccharide (LPS) is the major component of the outer membrane, and LPS molecules form a tightly packed barrier to exclude many antibiotics and detergents. Understanding and targeting LPS biosynthesis are crucial for developing novel antibiotics to break through the outer membrane and kill Gram-negative bacteria. Before being assembled in the outer membrane, LPS has to travel across the inner membrane, periplasm and outer member. This remarkable journey is powered by two ATP-binding cassette (ABC) transporters: MsbA and LptB2FGC. We have used single-particle cryo-EM and functional assays to reveal the mechanisms of these fascinating molecular machines.