Main > News and Events
As bacterial infections impervious to drugs rise, so does the need to develop better antibiotics
As long as antibiotics have existed, so too has antibiotic resistance—the inevitable result as infectious bacteria continually evolve to evade the very drugs designed to kill them.
Today, antibiotic resistance is considered a major global health threat. In the United States, The Centers for Disease Control and Prevention estimates that every year, at least 2.8 million people develop infections resistant to antibiotics, leading to more than 35,000 deaths.
Yet, in recent decades, antibiotic development has been slow, and no new classes of antibiotics have reached the market. Meanwhile, widespread use of the limited number of antibiotics currently available has spurred more bacterial strains to develop resistance, with additional strains already resistant to available antibiotics being discovered, often in hospitals. This situation is expected only to worsen over time, resulting in more drug-resistant bacterial infections and deaths.
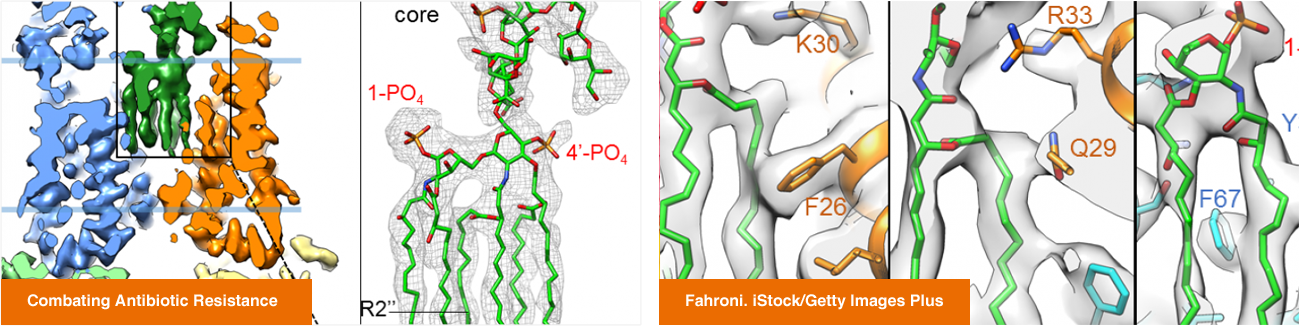
Maofu Liao, an associate professor of cell biology in the Blavatnik Institute at Harvard Medical School, spoke with Harvard Medicine News about antibiotic resistance and the challenges of developing new antibiotics.
Liao explained how his team’s research on protein structures in bacteria could inform antibiotic design and described a new pipeline his lab is establishing to improve the process.
In a newly published study in Science, Liao and colleagues demonstrated that their pipeline can effectively identify compounds that interfere with essential proteins in bacteria and thus may have potential as antibiotics.
HMNews: What are some of the most pressing challenges with currently available antibiotics?
Liao: One issue is that most drug development efforts depend on industry, but antibiotics are time-consuming and expensive to develop—and often aren’t necessarily required for treatment and aren’t taken by patients on a regular basis. It’s hard to make the business case to industry that it’s worthwhile to develop new antibiotics when so much effort and money are required, and profit isn’t predictable or immediate.
A second issue is the way we use antibiotics. For a long time, we have relied on single-use antibiotics, or a limited combination of antibiotics. This makes it easy for bacteria to acquire resistance. They can then transfer that capability to other bacteria that have not been exposed to antibiotics. So, we are using very limited tools that bacteria can easily overcome.
Another critical issue is how we develop antibiotics. With few exceptions, our efforts to identify new antibiotics mostly rely on chemical screens against bacterial growth. People do a screen and hope to find some magical compound that can kill the bacteria with great efficacy. Once they have that, they hand the compound to chemists who optimize it and hopefully develop it into a clinically useful antibiotic.
Such screens cannot target specific proteins in bacteria, and may exclude compounds that have the potential to attack crucial bacterial proteins. Moreover, for antibiotics developed through such screens, we often don’t know the underlying mechanism of how they work, or why they stop working when resistance occurs. This is a critical gap in our current approach.